Multiple Choice
Which of the following combinations of quantum numbers is permissible?
A) n = 1,l = 2,ml = 0,ms = 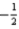
B) n = 3,l = 2,ml = 1,ms = 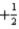
C) n = 3,l = 3,ml = 1,ms = 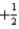
D) n = 2,l = 1,ml = -1,ms = 0
E) n = 4,l = 3,ml = 4,ms = 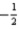
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: The contribution for which de Broglie is
Q37: What is the wavelength of a photon
Q38: How many p orbitals are in the
Q39: If the x-component of the velocity of
Q40: The angular momentum quantum number is best
Q42: A laser emits photons having an energy
Q43: What is the frequency of photons that
Q44: What is the value of the angular
Q45: If the location of a particular electron
Q46: Which quantum number distinguishes the different shapes