Multiple Choice
Which of the following statements is incorrect?
A) The set of quantum numbers n = 3,l = 2,ml = 0,ms = 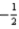 is not permitted because ml = 0.
is not permitted because ml = 0.
B) The set of quantum numbers n = 2,l = 2,ml = 1,ms = 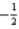 is not permitted because n = l.
is not permitted because n = l.
C) The set of quantum numbers n = 3,l = 2,ml = 1,ms = 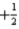 is permitted.
is permitted.
D) The set of quantum numbers n = 3,l = 2,ml = 3,ms = 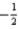 is not permitted because ml exceeds l.
is not permitted because ml exceeds l.
E) The set of quantum numbers n = 4,l = 3,ml = -1,ms = 0 is not permitted because ms = 0.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Based on the photoelectric effect,Einstein proposed the
Q3: All the following statements about the quantum
Q4: Rank the following regions of the electromagnetic
Q5: From the Bohr model of the hydrogen
Q6: What is the frequency of light emitted
Q7: Who postulated that energy is radiated only
Q8: What is the wavelength of an electron
Q9: The _ of a wave is the
Q10: What is the energy of a photon
Q11: Which of the following statements is incorrect?<br>A)The