Multiple Choice
The Pauli exclusion principle states that
A) the wavelength of a photon of light times its frequency is equal to the speed of light.
B) no two electrons in the same atom can have the same set of four quantum numbers.
C) both the position of an electron and its momentum cannot be known simultaneously very accurately.
D) the wavelength and mass of a subatomic particle are related by 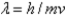 .
.
E) an electron can have either particle character or wave character.
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Which ground-state electron configuration is incorrect?<br>A)Br:[Ar]3d<sup>10</sup>4s<sup>2</sup>4p<sup>5</sup><br>B)K:[Ar]4s<sup>1</sup><br>C)Fe:[Ar]3d<sup>5</sup><br>D)Na:1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>1</sup><br>E)Zn:[Ar]3d<sup>10</sup>4s<sup>2</sup>
Q24: Which of the following statements is true
Q25: What is the maximum number of electrons
Q26: Which of the following ground-state electron configurations
Q27: An element that has the same ground
Q29: When arranged in order of increasing atomic
Q30: Which of the following atoms is paramagnetic
Q31: Mendeleev's periodic table (1869)can be explained by
Q32: Rank the following atoms in order of
Q33: Which element forms the most acidic oxide?<br>A)B<br>B)Tl<br>C)Al<br>D)In<br>E)Ga