Multiple Choice
Which of the following sets of four quantum numbers (n,l,ml, ms) correctly describes an electron occupying a d orbital of an element in the third row of the transition metals?
A) 4 2 -1 - 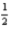
B) 5 2 2 + 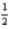
C) 5 3 -1 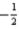
D) 4 1 0 - 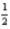
E) 5 0 0 + 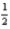
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Which of the following orbital diagrams represents
Q58: An atom of which of the following
Q59: All of the following ground-state electron configurations
Q60: An atom of which of the following
Q61: What is the ground-state electron configuration of
Q63: The elements that are filling the 5f
Q64: An atom of which of the following
Q65: What noble gas core would be used
Q66: Which principle or rule is violated by
Q67: An atom of which of the following