Multiple Choice
In the Born-Haber cycle for NaCl(s) ,which of the following processes corresponds to the enthalpy of formation of NaCl(s) ?
A) Na(s) + 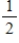 Cl2(g) → NaCl(s)
Cl2(g) → NaCl(s)
B) Na+(aq) + 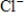 (aq) → NaCl(s)
(aq) → NaCl(s)
C) NaCl(g) → NaCl(s)
D) 2Na(g) + Cl2(g) → 2NaCl(g)
E) NaCl(aq) → NaCl(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q98: Which of the following bonds would be
Q99: Consider the reaction<br>2HCl(g)→ H<sub>2</sub>(g)+ Cl<sub>2</sub>(g); ΔH =
Q100: Which of the following compounds would be
Q101: What is the ground-state electron configuration of
Q102: In which of the following lists do
Q104: The total number of valence electrons in
Q105: Which of the following are two appropriate
Q106: The formation of which monatomic ion of
Q107: The formulas of many binary covalent compounds
Q108: In which pair do both compounds exhibit