Multiple Choice
Which of the following processes is not exothermic?
A) Rb+(g) + e- → Rb(g)
B) Rb+(g) + Cl-(g) → RbCl(s)
C) Cl(g) + e- → Cl-(g)
D) Rb(g) → Rb(s)
E) 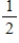 Cl2(g) → Cl(g)
Cl2(g) → Cl(g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: How many valence electrons does a carbonate
Q23: Which of the following Lewis formulas is
Q24: Based on the following data,what is the
Q25: The total number of valence electrons in
Q26: Rank the following ions in order of
Q28: Using bond-energy data,what is ΔH° for the
Q29: Which of the following is a correct
Q30: How many valence electrons are there in
Q31: The number of valence electrons in the
Q32: What is the total number of valence