Multiple Choice
Which of the following is the Lewis dot structure for the fluoride ion?
A) 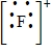
B) 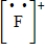
C) 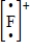
D) 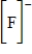
E) 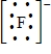
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: During the formation of a chemical bond
Q92: What is the ground-state electron configuration of
Q93: In which of the following species is
Q94: The following representation of an atom is
Q95: Which pair of species is isoelectronic?<br>A)Na<sup>+</sup> and
Q97: Which of the following diatomic molecules has
Q98: Which of the following bonds would be
Q99: Consider the reaction<br>2HCl(g)→ H<sub>2</sub>(g)+ Cl<sub>2</sub>(g); ΔH =
Q100: Which of the following compounds would be
Q101: What is the ground-state electron configuration of