Multiple Choice
The following species, 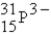 ,
, 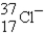
,and 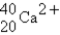
,all have the same number of
A) electrons.
B) nucleons.
C) neutrons.
D) protons.
E) isotopes.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Which pair of elements would form a
Q84: In the Lewis formula for phosphine,PH<sub>3</sub>,the number
Q85: The Lewis formula of which species does
Q86: What is the total number of valence
Q87: For the resonance hybrid of the nitrite
Q89: The Lewis structure for each of the
Q90: Atoms of an element X have the
Q91: During the formation of a chemical bond
Q92: What is the ground-state electron configuration of
Q93: In which of the following species is