Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 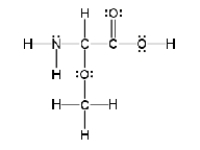
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q40: What is the predicted H-N-H bond angle
Q41: Which molecule or ion does not have
Q42: Which molecule or ion does not have
Q43: Which molecule or ion is not planar?<br>A)XeF<sub>4</sub><br>B)NO<sub>3</sub><sup>-</sup><br>C)BCl<sub>3</sub><br>D)F<sub>2</sub>CCF<sub>2</sub><br>E)CF<sub>4</sub>
Q44: For which molecule or ion does the
Q46: The molecular geometry of the ammonium ion,NH<sub>4</sub><sup>+</sup>,is
Q47: The approximate H-C-C bond angle in ethane,C<sub>2</sub>H<sub>6</sub>,is<br>A)60°.<br>B)180°.<br>C)120°.<br>D)109°.<br>E)90°.
Q48: Which molecule or ion has the highest
Q49: Which of the following species has(have)a bond
Q50: What is the molecular geometry around carbon