Multiple Choice
Given the molecular orbital diagram for dinitrogen (N2) excluding the K shells below,which of the following molecules or ions is expected to be diamagnetic? 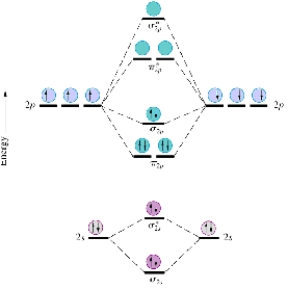
A) C22-
B) O2
C) B2
D) O2-
E) O2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Which molecule or ion has a trigonal
Q56: Which of the following statements is incorrect
Q57: The nitrosyl ion,NO<sup>+</sup>,has ten bonding electrons and
Q58: What is the hybridization of the nitrogen
Q59: In ClF<sub>3</sub>,the electron pairs are arranged about
Q61: A π bond is the result of
Q62: The following statements concern molecules that require
Q63: The molecular geometry of the CH<sub>3</sub><sup>+</sup> ion
Q64: Which molecule or ion does not have
Q65: What is the molecular geometry of the