Multiple Choice
Given the molecular orbital diagram for dinitrogen (N2) excluding the K shells below and assuming all species have a similar ordering of their MO's,which of the following would be expected to be diamagnetic? 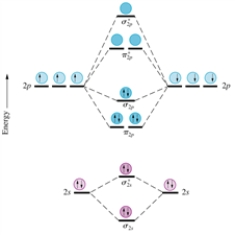
A) O2
B) F2−
C) O22−
D) B2
E) Li2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Which molecule or ion does not have
Q33: Which of the following statements correctly describes
Q34: The molecular geometry of the nitrite ion,NO<sub>2</sub><sup>-</sup>
Q35: Which molecule or ion has a trigonal
Q36: What is the hybridization of Br in
Q38: Which of the following molecules is nonpolar?<br>A)
Q39: Which of the following statements concerning ozone
Q40: What is the predicted H-N-H bond angle
Q41: Which molecule or ion does not have
Q42: Which molecule or ion does not have