Multiple Choice
Consider the following series of molecular ions and molecules: F2+,F22+,F2,and F2-.Which will have the shortest bond length between the fluorine atoms? Assume the homonuclear molecular orbital diagram provided below for nitrogen (excluding the K shells) still applies to these species. 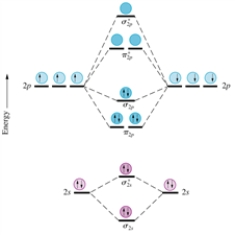
A) F2+
B) The bond lengths are all equivalent.
C) F22+
D) F2
E) F2-
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which of the following electron distributions among
Q7: Which one of the following statements provides
Q8: What is the molecular geometry around an
Q9: What is the H-O-H bond angle in
Q10: Which molecule is polar?<br>A)C<sub>2</sub>H<sub>4</sub><br>B)CS<sub>2</sub><br>C)C<sub>6</sub>H<sub>6</sub><br>D)SO<sub>2</sub><br>E)CF<sub>4</sub>
Q12: Which of the following statements is not
Q13: Which molecule or ion has a bond
Q14: Which of the following molecules has a
Q15: What is the molecular geometry around oxygen
Q16: In phosgene,COCl<sub>2</sub>,the electron groups are located about