Multiple Choice
The enthalpy of fusion of aluminum is 10.7 kJ/mol.How many grams of aluminum can be melted by adding 81.4 kJ of energy to the metal at its melting point?
A) 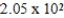 g
g
B) 32.2g
C) 3.01 g
D) 7.6 g
E) 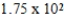 g
g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: The metal rubidium crystallizes in a body-centered
Q48: Which of the following pure substances has
Q49: Which of the following is a molecular
Q50: The number of nearest-neighbor atoms of an
Q51: Which of the following substances has the
Q53: Which of the following is the strongest
Q54: The process represented by the equation C<sub>10</sub>H<sub>8</sub>(s)→
Q55: Which of the following statements concerning liquids
Q56: Which substance can be described as cations
Q57: Van der Waals forces must be broken