Multiple Choice
The space-filling representation of a crystalline potassium provided below is an example of a _____ unit cell,which contains the equivalent of _____ atom(s) within a single unit cell.
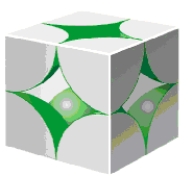
A) body centered cubic,2 atoms
B) face centered cubic,4 atoms
C) simple cubic,1 atom
D) body centered cubic,4 atoms
E) face centered cubic,5 atoms
Correct Answer:

Verified
Correct Answer:
Verified
Q112: Which of the following pure substances has
Q113: A metal crystallizes in a face-centered cubic
Q114: If more ice is added to an
Q115: Vanadium crystallizes in the body-centered cubic system.If
Q116: Which bonding interaction best describes the strongest
Q118: Lithium chloride crystallizes in a face-centered cubic
Q119: The strongest intermolecular forces between molecules of
Q120: Lithium chloride crystallizes in a face-centered cubic
Q121: Which of the following compounds has the
Q122: Which of the following compounds is expected