Multiple Choice
Nickel crystallizes in a face-centered cubic lattice.The density of the element is 8.91 g/cm3.What is the volume of a single unit cell?
A) 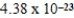 cm3
cm3
B) 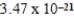 cm3
cm3
C) 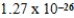 cm3
cm3
D) 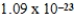 cm3
cm3
E) 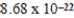 cm3
cm3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: In a certain mountain range,water boils at
Q20: Which of the following pure substances has
Q21: Which of the following best describes calcium
Q22: The metal sodium crystallizes in a body-centered
Q23: Which of the following pure substances has
Q25: If the diameter of a spherical water
Q26: Why does hydrogen fluoride have an unusually
Q27: What is the value of q when
Q28: Identify the van der Waals equation.<br>A) <img
Q29: Platinum crystallizes with a face-centered cubic unit