Multiple Choice
Consider the following gas-liquid equilibrium for an aqueous system at a constant partial pressure of N2.
N2(g) 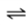
N2(aq)
What is the effect on the equilibrium composition of the liquid when the temperature of the liquid is increased?
A) The amount of N2 dissolved in the liquid increases.
B) The amount of N2 dissolved in the liquid decreases.
C) The amount of N2 dissolved in the liquid does not change.
D) Not enough information is provided to answer the question.
E) Either A or B could occur.
Correct Answer:

Verified
Correct Answer:
Verified
Q68: A cucumber is placed in a concentrated
Q69: What is the molarity of a 10.0%
Q70: What is the vapor pressure at 75°C
Q71: Determine the osmotic pressure of a solution
Q72: What is the molality of a solution
Q74: If two fluids do not mix but,rather,form
Q75: Which of the following methods cannot be
Q76: Which of the following is not a
Q77: A concentrated ammonia solution is 28.0% NH<sub>3</sub>
Q78: What is the mole fraction of water