Multiple Choice
According to the National Institute of Standards webbook,the Henry's Law constant for N2 gas is 0.000639 mol/kg⋅bar at 25°C What is the Henry's law constant in units of mol/kg⋅atm? (1 bar = 0.9869 atm; 1 atm = 760 mmHg)
A) 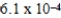 × 10-4 mol/kg⋅atm
× 10-4 mol/kg⋅atm
B) 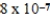 × 10-7 mol/kg⋅atm
× 10-7 mol/kg⋅atm
C) 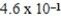 × 10-1 mol/kg⋅atm
× 10-1 mol/kg⋅atm
D) 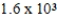 × 103 mol/kg⋅atm
× 103 mol/kg⋅atm
E) 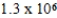 × 106 mol/kg⋅atm
× 106 mol/kg⋅atm
Correct Answer:

Verified
Correct Answer:
Verified
Q88: What is the mole fraction of toluene
Q89: A 12.0% sucrose solution by mass has
Q90: What type of colloid is formed when
Q91: If 11.2 g of naphthalene,C<sub>10</sub>H<sub>8</sub>,is dissolved in
Q92: For a dilute solution of MgCl<sub>2</sub>,the van't
Q94: How many moles of urea (60.g/mol)must be
Q95: What mass of an aqueous 33.7% sodium
Q96: A suspension of silver particles in water
Q97: At a particular temperature the solubility of
Q98: What is the freezing point of an