Multiple Choice
The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 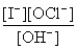
) If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
A) mol2/(L2 ∙ s)
B) L/(mol ∙ s)
C) 1/s
D) L2/(mol2 ∙ s)
E) mol/(L ∙ s)
Correct Answer:

Verified
Correct Answer:
Verified
Q20: A suggested mechanism for the decomposition of
Q21: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The Arrhenius
Q22: For a certain reaction of the general
Q23: The rate law for the hydrolysis of
Q24: The reaction CHCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The
Q26: The reaction A → products is first-order
Q27: The rate law for the reaction between
Q28: What would happen if the kinetic energy
Q29: Which of the following statements is incorrect
Q30: The following data were obtained in a