Multiple Choice
Which of the following is not a correct representation of the integrated rate expression for a first-order reaction?
A) 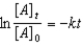
B) 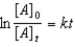
C) 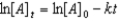
D) 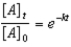
E) 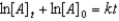
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: Which of the following statements is incorrect?<br>A)The
Q99: The catalyzed pathway in a reaction mechanism
Q100: The hypochlorite ion oxidizes the iodide ion
Q101: The nuclide <sup>96</sup>Nb decays by a first-order
Q102: A possible mechanism for the gas phase
Q104: At a given temperature,a first-order reaction has
Q105: For the first-order reaction<br>1/2 N<sub>2</sub>O<sub>4</sub>(g)→ NO<sub>2</sub>(g); ΔH
Q106: The half-life of a reaction is<br>A)twice as
Q107: Which of the following reactions is not
Q108: For the hypothetical reaction A → products,the