Multiple Choice
Which of the following corresponds to the correct equation for the half-life of a first-order reaction?
A) 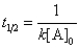
B) 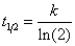
C) 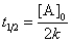
D) 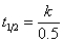
E) 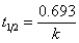
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: What would happen if the kinetic energy
Q29: Which of the following statements is incorrect
Q30: The following data were obtained in a
Q31: The rate law for a reaction is
Q32: For the formation of 1 mol of
Q34: For the following reaction producing 1 mol
Q35: A proposed mechanism for the decomposition of
Q36: For the hypothetical reaction aA → products,the
Q37: The rate constant for a first-order reaction
Q38: Determine the molecularity of the following elementary