Multiple Choice
For the hypothetical reaction aA → products,the concentration of A was monitored with time.Given the following graph of the experimental data,what is the rate constant for the loss of reactant A? 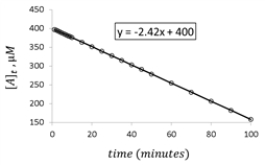
A) 2.42 μΜ −1⋅min−1
B) 400.00 μΜ −1⋅min−1
C) -2.42 μΜ −1⋅min−1
D) -400 μΜ −1⋅min−1
E) 24.2 μΜ −1⋅min−1
Correct Answer:

Verified
Correct Answer:
Verified
Q74: Which of the following statements best describes
Q75: The reaction between selenous acid and the
Q76: For which of the following hypothetical rate
Q77: Ozone reacts with nitrogen dioxide to produce
Q78: If a reaction is first-order with respect
Q80: The rates of most chemical reactions are
Q81: The reaction<br>2H<sub>2</sub>(g)+ 2NO(g)→ 2H<sub>2</sub>O(g)+ N<sub>2</sub>(g)<br>Is first-order in
Q82: For a certain reaction of the general
Q83: A reaction that is second-order in one
Q84: The nuclide <sup>188</sup>W decays by a first-order