Multiple Choice
How many mechanistic steps are depicted by in this potential energy diagram for the decomposition of cyclobutane to ethylene? 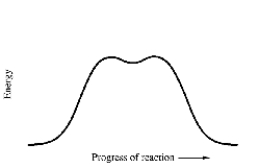
A) one step
B) two steps
C) three steps
D) four steps
E) five steps
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Dinitrogen tetroxide decomposes to form nitrogen dioxide
Q2: The following mechanism has been suggested for
Q3: For the reaction of the ammonium ion
Q4: In a chemical reaction at constant temperature,the
Q5: The oxidation of ammonia produces nitrogen and
Q7: In aqueous solution,iodine reacts with acetone as
Q8: The complete mechanism for a reaction is
Q9: For the reaction<br>6CH<sub>2</sub>O(aq)+ 4NH<sub>3</sub>(aq)→ (CH<sub>2</sub>)<sub>6</sub>N<sub>4</sub>(aq)+ 6H<sub>2</sub>O(l)<br>The rate
Q10: For a second-order reaction,what are the possible
Q11: Identify the rate equation of the