Multiple Choice
What is the reaction quotient (Q) for the equilibrium
TlSCN(s) 
Tl+(aq) + SCN-(aq)
When 0.1837 L of 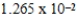
M Tl+ is combined with 0.1335 L of 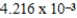
M SCN- in the presence of an excess of TlSCN(s) ?
A) 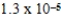
B) 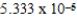
C) 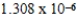
D) 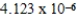
E) 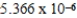
Correct Answer:

Verified
Correct Answer:
Verified
Q60: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="For
Q61: The _ is an economical process for
Q62: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="For
Q63: When cobalt chloride is added to pure
Q64: Carbon tetrachloride may react with oxygen to
Q66: For the reaction Br<sub>2</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q67: At 800 K,K<sub>c</sub> for the following equilibrium
Q68: Which of the following statements is incorrect
Q69: A sample of ammonia gas was allowed
Q70: For the following reaction system at equilibrium,which