Multiple Choice
Which of the following equilibria would not be affected by pressure changes at constant temperature?
A) CO2(g) + H2(g)  CO(g) + H2O(g)
CO(g) + H2O(g)
B) CO(g) + 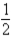 O2(g)
O2(g)  CO2(g)
CO2(g)
C) 2Hg(l) + O2(g)  2HgO(s)
2HgO(s)
D) 2H2(g) + O2(g)  2H2O(l)
2H2O(l)
E) CaCO3(s)  CaO(s) + CO2(g)
CaO(s) + CO2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For the equilibrium N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="For
Q2: What balanced equation is the following equilibrium
Q3: For which of the following systems at
Q4: Consider the following equilibrium:<br>2NO(g)+ 3F<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q6: What is the expression for K<sub>c</sub> for
Q7: One method for the decomposition of carbon
Q8: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q9: Which of the following equilibria would be
Q10: Solid HgO,liquid Hg,and gaseous O<sub>2</sub> are placed
Q11: Which of the following statements is true