Multiple Choice
At a temperature of 25°C an initally 0.011 M solution of a weak monoprotic acid is 2.9 % ionized once equilibrium is established.What is the acid-ionization constant,Ka,for this acid? (assume Ca/Ka ≥ 102)
A) 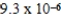
B) 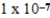
C) 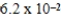
D) 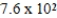
E) 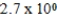
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: What is the hydronium-ion concentration of a
Q82: What is the base-ionization equilibrium constant for
Q83: What is the pH of a buffer
Q84: Which of the following statements is true
Q85: What is the equilibrium concentration of ammonium
Q87: What is the pH of a 0.24
Q88: A 75.0-mL sample of 0.0500 M HCN
Q89: What molar ratio of acetic acid to
Q90: What will happen if a small amount
Q91: What is the hydroxide-ion concentration of a