Multiple Choice
An initially 1.0 M aqueous solution of a weak monoprotic acid has a total ion concentration of 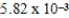 M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
A) 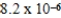
B) 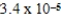
C) 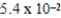
D) 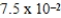
E) 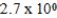
Correct Answer:

Verified
Correct Answer:
Verified
Q48: What is K<sub>c</sub> for the following equilibrium?
Q49: What is the pH of a solution
Q50: What is the hydronium-ion concentration of a
Q51: What is K<sub>a</sub> at 25°C for the
Q52: Which of the following substances,if added to
Q54: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q55: In a 0.01 M solution of 1,4-butanedicarboxylic
Q56: What is the hydronium-ion concentration of a
Q57: Suppose a buffer solution is made from
Q58: The oxygen-carrying function of blood depends on