Multiple Choice
The equilibrium hydronium ion concentration of an initially 0.655 M solution of a monoprotic weak acid is  M.The acid dissociation constant is
M.The acid dissociation constant is 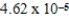
At 25°C.What is the pH of this solution?
A) 2.26
B) 7.00
C) 11.74
D) 4.52
E) 0.18
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q126: Which of the following solutions has the
Q127: Consider the titration of 300.0 mL of
Q128: A sample of ammonia (K<sub>b</sub> = 1.8
Q129: Which of the following mixtures will be
Q130: What is the pOH of a solution
Q132: For which of the following equilibria does
Q133: A weak base is titrated with a
Q134: Which of the following indicators is most
Q135: Carbonic acid,H<sub>2</sub>CO<sub>3</sub>,is a weak diprotic acid.In a
Q136: What is the pOH of a solution