Multiple Choice
What is the equilibrium percent ionization of an initially 0.32 M solution of the monoprotic acid valeric acid,HC5H9O2,at 25°C (Ka = 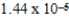 ) ?
) ?
A) 0.67%
B) 1.30%
C) 0.0014%
D) 0.1%
E) 2.00%
Correct Answer:

Verified
Correct Answer:
Verified
Q138: What is the hydronium-ion concentration in a
Q139: For which of the following equilibria does
Q140: In a 0.20 M solution of a
Q141: Which of the following statements is true
Q142: What is the hydroxide-ion concentration at equilibrium
Q143: For a 0.05 M H<sub>2</sub>SO<sub>3</sub> solution,which of
Q145: What mass of sodium hydroxide must be
Q146: Given the following,what will be the approximate
Q147: What is the equilibrium expression for the
Q148: Rank acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>),hydrocyanic acid (HOCN),and hydrofluoric