Multiple Choice
When equal volumes of solutions are mixed,as shown below,what will be the relative pH values of the resulting solutions? 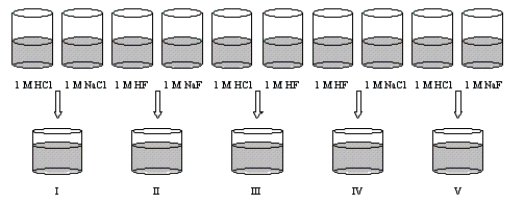
A) I < V < III < II < IV
B) V < I < IV < II < III
C) III < I < IV = V < II
D) I = V < III < II < IV
E) III < I = V < III < IV < II
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: What is the percent ionization of a
Q27: A 0.10 M solution of a weak
Q28: For the equilibrium that exists in an
Q29: Which of the following salts will produce
Q30: A 0.010 M aqueous solution of a
Q32: Which of the following combinations is not
Q33: What is the concentration of HC<sub>2</sub>O<sub>4</sub><sup>-</sup> in
Q34: What is the pH of a solution
Q35: It is safe to make the simplifying
Q36: A certain weak base B has a