Multiple Choice
Cation C and anion A form an ionic compound for which Ksp = s2,where s is the molar solubility of the ionic compound.Which of Figures I-III represent(s) possible results of the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A? 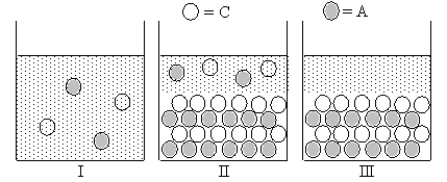
A) only I
B) only III
C) both I and III
D) both I and II
E) only II
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Cation C and anion A form an
Q21: What is the molar solubility of MgF<sub>2</sub>
Q22: Which of the following salts has the
Q23: K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 ×10<sup>-8</sup>.If a
Q24: The figure below represents the result of
Q26: What is the molar equilibrium concentration of
Q27: The solubility of calcium carbonate in water
Q28: Pure water is saturated with slightly soluble
Q29: The following reaction represents a step in
Q30: Which of the following solutions should be