Multiple Choice
Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s) that represent(s) products for which Ksp = s2,where s is the molar solubility of the ionic compound. 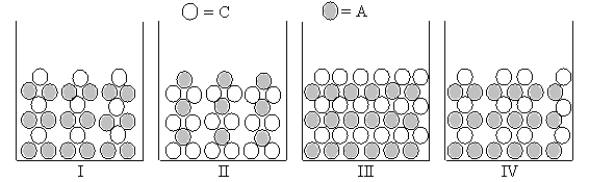
A) only I
B) only II
C) only IV
D) only III
E) both I and II
Correct Answer:

Verified
Correct Answer:
Verified
Q12: A 5.0 × 10<sup>-4</sup> M solution of
Q13: What is the concentration of Cd<sup>2+</sup> in
Q14: A saturated solution of which of the
Q15: If 500 mL of 1.3 × 10<sup>-6</sup>
Q16: What is the correct mathematical expression for
Q18: Which of the following insoluble salts will
Q19: What is the molar solubility of nickel(II)sulfide
Q20: Cation C and anion A form an
Q21: What is the molar solubility of MgF<sub>2</sub>
Q22: Which of the following salts has the