Multiple Choice
The solubility of silver(I) carbonate is 3.6 × 10-2 g/L.What is the solubility product constant for silver(I) carbonate?
A) 4.4 × 10-15
B) 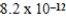
C) 1.7 × 10-8
D) 1.3 × 10-4
E) 1.3 × 10-3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: What is the molar solubility of silver(I)chloride
Q54: Which of the following salts has the
Q55: Rank the following salts in order of
Q56: Which of Figures I-IV represent(s)the result of
Q57: What is the maximum volume of 8.6
Q59: Consider a solution containing the following cations:
Q60: What is the molar solubility of lead(II)chloride
Q61: A _ is an ion formed from
Q62: What is the molar solubility of zinc
Q63: The following reaction represents a step in