Multiple Choice
Rank the following salts in order of increasing molar solubility.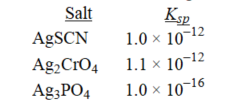
A) AgSCN < Ag2CrO4 < Ag3PO4
B) AgSCN < Ag3PO4 < Ag2CrO4
C) Ag3PO4 < Ag2CrO4 < AgSCN
D) Ag3PO4 < AgSCN < Ag2CrO4
E) Ag2CrO4 < AgSCN < Ag3PO4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: The solubility of calcium carbonate in water
Q28: Pure water is saturated with slightly soluble
Q29: The following reaction represents a step in
Q30: Which of the following solutions should be
Q31: In the qualitative analysis scheme for metal
Q33: A(n)_ is a Lewis base that bonds
Q34: What is the molar solubility of MgF<sub>2</sub>
Q35: The solubility of lead(II)sulfate is 4.0 ×
Q36: Which of the following salts has the
Q37: Which sparingly soluble salt will exhibit the