Multiple Choice
What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 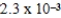 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
A) 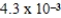 M
M
B) 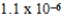 M
M
C) 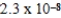 M
M
D) 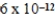 M
M
E) 2.6 × 10-14 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Which salt has the lowest molar solubility
Q44: What is the effect of substituting soluble
Q45: A saturated solution of which of the
Q46: Determine the molar solubility of AgCl in
Q47: Which of the following will apply to
Q49: What is the value of the dissociation
Q50: What is the molar solubility of aluminum
Q51: A solution is 0.010 M in each
Q52: What is the solubility product expression for
Q53: What is the molar solubility of silver(I)chloride