Multiple Choice
The figure below represents the results of adding a strong acid to a saturated solution of an ionic compound.Which of the following could be the ionic compound? 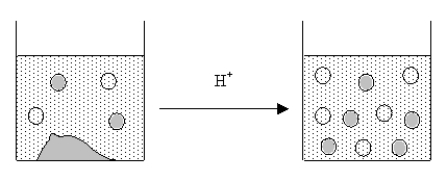
A) AgClO4
B) AgF
C) AgI
D) AgCl
E) AgBr
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Figures I-IV represent ionic compounds formed upon
Q2: To 1.0 L of water,1.5 × 10<sup>-6</sup>
Q3: For which of the following salts would
Q5: Which of the following substances will increase
Q6: What is the solubility (in g/L)of silver(I)chloride
Q7: For which pair of cations would the
Q8: What will happen if 50.0 mL of
Q9: A solution contains 0.018 mol each of
Q10: What is the minimum concentration of Pb<sup>2+</sup>
Q11: What is the solubility (in g/L)of aluminum