Multiple Choice
The figure below represents the results of adding NH3 to a saturated solution of an ionic compound.Which of the following could the ionic compound be? 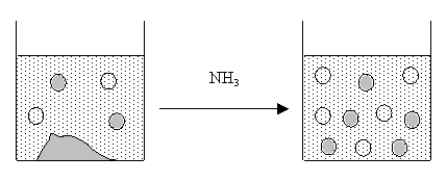
A) AgCl or CaF2
B) CaF2
C) Mg(OH) 2
D) AgCl
E) SrCO3
Correct Answer:

Verified
Correct Answer:
Verified
Q77: After mixing an excess PbCl<sub>2</sub> with a
Q78: What is the solubility product expression for
Q79: What is the molar solubility of lead(II)sulfate
Q80: Which of the following is not likely
Q81: In the sulfide scheme for qualitative analysis,the
Q83: The insoluble salts AV,B<sub>2</sub>W,C<sub>2</sub>X<sub>3</sub>,DY<sub>2</sub>,and EZ<sub>3</sub>,which were formed
Q84: Suppose 50.00 mL of a 1 ×
Q85: If 275 mL of 1 × 10<sup>-7</sup>
Q86: What is the maximum hydroxide-ion concentration that
Q87: Sodium chloride is added slowly to a