Multiple Choice
oWhat is the change in free energy at 298 K when 70.0 mL of 0.704 M calcium chloride is combined with 47.9 mL of 0.859 M sodium carbonate? (R = 8.31 J/(K ∙ mol) )
Ca2+(aq) + CO32-(aq) → CaCO3(s) 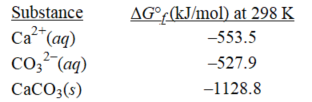
A) 42.6 kJ
B) -2.21 × 103 kJ
C) 46.1 kJ
D) 47.4 kJ
E) 2.56 × 103 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q35: For which of the following processes would
Q36: What is ΔG° at 298 K for
Q37: For a reversible phase change at constant
Q38: Which of the following is the best
Q39: Condensation is a process for which<br>A)ΔG is
Q41: The following reaction is spontaneous at all
Q42: Determine ΔG° for the following reaction:<br>CH<sub>4</sub>(g)+ 2O<sub>2</sub>(g)
Q43: Consider the following reaction:<br>2C(s)+ 2H<sub>2</sub>(g)→ C<sub>2</sub>H<sub>4</sub>(g); ΔH°
Q44: What is the change in internal energy
Q45: An ideal fuel for the control jet