Multiple Choice
Based on the following data,what is the standard Gibbs free energy of formation of the sulfate ion at 298 K? (R = 8.31 J/(K ∙ mol) )
PbSO4(s)  Pb2+(aq) + SO42-(aq) ; Ksp = 1.7 × 10-8
Pb2+(aq) + SO42-(aq) ; Ksp = 1.7 × 10-8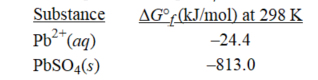
A) -788.6 kJ/mol
B) -793.1 kJ/mol
C) -837.4 kJ/mol
D) -744.3 kJ/mol
E) 832.9 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Consider the following reaction,which is spontaneous at
Q59: The third law of thermodynamics states that<br>A)the
Q60: For the reaction MgSO<sub>3</sub>(s)→ MgO(s)+ SO<sub>2</sub>(g),which is
Q61: What does the third law of thermodynamics
Q62: In which of the following scenarios is
Q64: Which of the following reactions has the
Q65: Which of the following equations is correct?<br>A)G
Q66: Under standard-state conditions,a reaction with ΔH° <
Q67: For the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g),ΔH° and
Q68: What is the change in entropy when