Multiple Choice
Given the following,determine K at 298K for the reaction,
AgI(s) → Ag+(aq) +I−(aq) 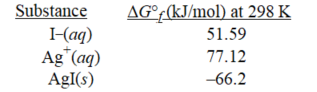
A) 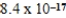
B) 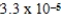
C) 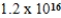
D) 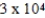
E) 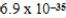
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Consider the following reaction:<br>2C(s)+ 2H<sub>2</sub>(g)→ C<sub>2</sub>H<sub>4</sub>(g); ΔH°
Q44: What is the change in internal energy
Q45: An ideal fuel for the control jet
Q46: For which of the following reactions is
Q47: Which of the following has the lowest
Q49: A 0.0333 M solution of a particular
Q50: For the reaction N<sub>2</sub>(g)→ 2N(g),<br>A)ΔH < 0
Q51: What is the change in entropy when
Q52: The free-energy change of a reaction is
Q53: For which of the following reactions is