Multiple Choice
The cell potential of an electrochemical cell with the cell reaction
Al(s) + Cr3+(aq) → Cr(s) + Al3+(aq)
Is 1.63 V.What is the maximum electrical work obtainable from this cell when 0.50 g of Al is consumed?
A) 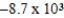 J
J
B) 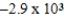 J
J
C) 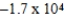 J
J
D) 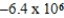 J
J
E) 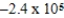 J
J
Correct Answer:

Verified
Correct Answer:
Verified
Q98: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q99: What is the cell notation for the
Q100: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q101: What is the half-reaction that occurs at
Q102: What is E°<sub>cell</sub> for the cell reaction
Q104: If an electrolysis plant operates its electrolytic
Q105: Molten magnesium chloride is electrolyzed using inert
Q106: Consider the following cell reaction:<br>2Cr(s)+ 6H<sup>+</sup>(aq)→ 2Cr<sup>3+</sup>(aq)+
Q107: Consider the following reduction potentials:<br>Cd<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img
Q108: Which reaction would be most likely to