Multiple Choice
A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 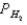 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
A) 0.41 V,Fe
B) 0.90 V,Pt
C) -0.41 V,Pt
D) -0.41 V,Fe
E) 0.41 V,Pt
Correct Answer:

Verified
Correct Answer:
Verified
Q10: In the following electrochemical cell,what is the
Q11: The process of producing a chemical change
Q12: What is the cell reaction for the
Q13: A piece of iron half-immersed in a
Q14: The cell potential of an electrochemical cell
Q16: An alkaline dry cell is similar to
Q17: If the cell is initially at standard-state
Q18: The _ has zinc can as the
Q19: Given:<br>W<sup>3+</sup>(aq)+ 3e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: W<sup>3+</sup>(aq)+ 3e<sup>-</sup>
Q20: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>