Multiple Choice
If E°cell for a certain reaction is 1 V (n = 4) ,and ΔS° is 11.2 J/(K⋅mol) ,what is ΔH° in units of J/mol at 25°C?
A) 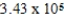 × 105 J/mol
× 105 J/mol
B) 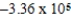 × 105 J/mol
× 105 J/mol
C) 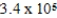 × 105 J/mol
× 105 J/mol
D) 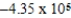 × 104 J/mol
× 104 J/mol
E) 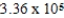 J/mol
J/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q89: In order to determine the identity of
Q90: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given:
Q91: What is the balanced spontaneous reaction and
Q92: A _ is a commercial electrochemical cell
Q93: If the value of E°<sub>cell</sub> is 2.10
Q95: Balance the following oxidation-reduction occurring in acidic
Q96: What is the cell reaction for the
Q97: Given:<br>Ni<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Ni<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q98: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q99: What is the cell notation for the