Multiple Choice
A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 3.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.10 M and 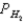 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
A) 0.10 V
B) 0.57 V
C) 0.27 V
D) 0.25 V
E) 0.56 V
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When the following oxidation-reduction reaction in acidic
Q3: A fuel cell designed to react grain
Q4: Given:<br>Mn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Mn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q5: For a galvanic cell using Fe |
Q6: What is the cell reaction for the
Q8: What is one of the major products
Q9: Given:<br>Li<sup>+</sup>(aq)+ e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Li<sup>+</sup>(aq)+ e<sup>-</sup>
Q10: In the following electrochemical cell,what is the
Q11: The process of producing a chemical change
Q12: What is the cell reaction for the