Multiple Choice
The following cell is initially at standard-state conditions.Which of the following statements is true after the cell is allowed to discharge (do work) for a period of time? 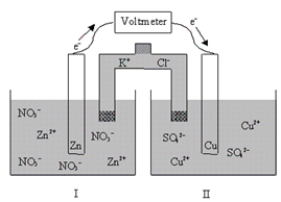 Zn2+(aq) + 2e-
Zn2+(aq) + 2e-  Zn(s) ; E° = -0.76 V
Zn(s) ; E° = -0.76 V
Cu2+(aq) + 2e-  Cu(s) ; E° = 0.34 V
Cu(s) ; E° = 0.34 V
A) Initially Ecell = - 1.10 V,and it will become more negative with time.
B) Ecell does not change with time.
C) Initially Ecell = - 1.10 V,and it will become more positive with time.
D) Initially Ecell = + 1.10 V,and it will become more negative with time.
E) Initially Ecell = + 1.10 V,and it will becomes more positive with time.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: In the following electrochemical cell,what is the
Q34: Which statement concerning the anode in an
Q35: A lead storage battery involves the following
Q36: Which of the following statements is true
Q37: When balancing oxidation-reduction reactions in acidic solution
Q39: The cell potential of the following cell
Q40: According to the following cell notation,which species
Q41: A strip of iron is placed in
Q42: Given:<br>Hg<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Hg<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q43: When the following oxidation-reduction reaction in acidic