Multiple Choice
After 3.2 hours, 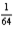 of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?
of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?
A) 32 min
B) 45 min
C) 60 min
D) 74 min
E) 20 min
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: When the radioactive nuclide <sup>110</sup>In undergoes positron
Q31: The first nuclear reaction that was ever
Q32: As a radioactive isotope decays,its rate constant<br>A)remains
Q33: In the ejection of a beta particle
Q34: What is the nuclear binding energy per
Q36: What is the abbreviated notation for the
Q37: Nuclides with too many neutrons to be
Q38: Which of the following statements is incorrect?<br>A)Isotope
Q39: A nuclear _ is a self-sustaining series
Q40: What is the mass defect of <sup>11</sup><sub>4</sub>Be?<br>Particle<br>Mass