Multiple Choice
What quantity of energy is released per gram of U-235 based on the following neutron induced fission of U-235? (c = 3.00 × 108 m/s) 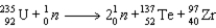 Particle
Particle
Mass (amu) 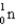
1) 008665 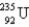
235) 043922 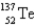
136) 9253 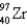
96) 910950
A) 7.62 × 1010 J/g U-235
B) 7.62 × 1013 J/g U-235
C) 1.79 × 1010 J/g U-235
D) 1.79 × 1013 J/g U-235
E) 3.10 × 1011 J/g U-235
Correct Answer:

Verified
Correct Answer:
Verified
Q36: What is the abbreviated notation for the
Q37: Nuclides with too many neutrons to be
Q38: Which of the following statements is incorrect?<br>A)Isotope
Q39: A nuclear _ is a self-sustaining series
Q40: What is the mass defect of <sup>11</sup><sub>4</sub>Be?<br>Particle<br>Mass
Q42: When the radioactive nuclide <sup>62</sup>Zn undergoes electron
Q43: A particular nuclear bombardment reaction is represented
Q44: When a nucleus undergoes radioactive decay,its new
Q45: A 4.50-mg sample of a newly discovered
Q46: A sodium nucleus, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="A sodium