Multiple Choice
The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800-1000°C) and high pressures (10-15 atm) with nickel as a catalyst. 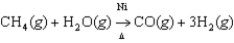 If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
A) 171 L
B) 1330 L
C) 249 L
D) 25.0 L
E) 222 L
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Which of the following statements concerning chlorine
Q59: Which of the following statements concerning magnesium
Q60: Which of the following statements about sodium
Q61: Which of the following statements about lithium
Q62: Which of the following combinations of common
Q64: The Group 8A elements in the periodic
Q65: Which of the following statements about compounds
Q66: Which of the following is not a
Q67: Polyphosphoric and metaphosphoric acids are made by
Q68: Dinitrogen gas is prepared commercially<br>A)from the decomposition