Multiple Choice
A complex ion is a square planar complex.It has a d8 electron configuration.What is the most reasonable d orbital scheme for this complex?
A) 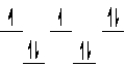
B) 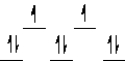
C) 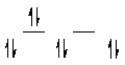
D) 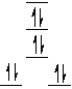
E) 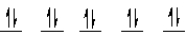
Correct Answer:

Verified
Correct Answer:
Verified
Q50: 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>3d<sup>2</sup> is the correct electron configuration for
Q51: Which of the following species is least
Q52: Which of the following high-spin complexes has
Q53: What is the oxidation state of iron
Q54: The spectrochemical series is an arrangement of<br>A)ligands
Q56: An element with the electron configuration [Xe]6s<sup>2</sup>4f<sup>14</sup>5d<sup>7</sup>
Q57: Fe has _ that is(are)unpaired in its
Q58: Which combination leads to a high-spin octahedral
Q59: Molecules that have nonsuperimposable mirror images are<br>A)multidentate.<br>B)racemic.<br>C)dextrorotatory.<br>D)chiral.<br>E)levorotatory.
Q60: The complexes [Ni(NH<sub>3</sub>)<sub>5</sub>Cl]Br and [Ni(NH<sub>3</sub>)<sub>5</sub>Br]Cl are what