Multiple Choice
Near a pH of 5.5,L-lysine (R = −(CH2) 4NH2) the major species in aqueous solution is the diprotonated zwitterion.Given the following acid dissociation constants,what is the correct structure of the zwitterion?
Functional Group
Ka
Carboxylic acid
1) 7 x10-2
Amine
8) 5 x10-10
Side chain amine
1) 51 x10-11
A) 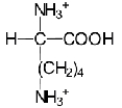
B) 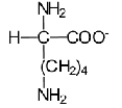
C) 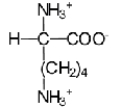
D) 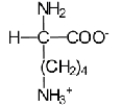
E) 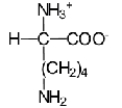
Correct Answer:

Verified
Correct Answer:
Verified
Q4: What is the type of polymer formed
Q5: What is the type of polymer formed
Q6: How many different tripeptides can be made
Q7: The alpha helix and beta sheet are
Q8: Which of the following units is not
Q10: Elimination of a small molecule such as
Q11: The nucleic acid sequence that is complementary
Q12: For the addition polymer polyacrylonitrile shown below,what
Q13: Polymers of amino-acid units are called<br>A)metabolites.<br>B)nucleic acids.<br>C)lipids.<br>D)carbohydrates.<br>E)proteins.
Q14: A hemiketal is formed from a(n)_.<br>A)alcohol and