Multiple Choice
System 1 is 3.1 moles of a monatomic ideal gas held at a constant volume of 2.51 L as it undergoes a temperature increase.System 2 is 3.1 moles of a diatomic ideal gas held at a constant volume of 2.51 L as it undergoes a temperature increase equal to the temperature increase of System 1.The pressure increase for system 1 is 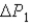
And for System 2 is 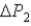
During the temperature increases.What is the ratio of 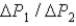
?
A) 1.00
B) 0.60
C) 1.67
D) 2.50
E) 4.50
Correct Answer:

Verified
Correct Answer:
Verified
Q13: A 1.0-kg chunk of ice at 0°C
Q23: In an isovolumetric process by an ideal
Q26: If one could observe the individual atoms
Q44: The Carnot cycle consists of a combination
Q60: A system is acted on by its
Q61: A heat engine operating between a pair
Q62: A pound of body fat has an
Q66: A closed 2.0-L container holds 3.0 mol
Q69: Three Carnot engines operate between temperature reservoirs
Q72: An adiabatic expansion refers to the fact